
ICCS S2-2
(LO)
Combined presentation with previous abstract
SOLIFENACIN IN CHILDREN AND ADOLESCENTS WITH OVERACTIVE BLADDER:
RESULTS OF AN OPEN-LABEL, LONG-TERM CLINICAL TRIAL
Brigitte BOSMAN
1
, Donald NEWGREEN
1
, Will SAWYER
2
, Adriana HOLLESTEIN-HAVELAAR
3
, Ellen DAHLER
3
, Stéphane
BOLDUC
4
and Soeren RITTIG
5
1) Astellas Pharma Europe BV, Global Medical Science - Urology / Nephrology, Leiden, NETHERLANDS - 2) Astellas
Pharma Europe BV, Global Development Operations Biostatistics II, Leiden, NETHERLANDS - 3) Astellas Pharma Europe
BV, Global Development Operations, Clinical Science, Leiden, NETHERLANDS - 4) CHU de Québec, Division of Urology,
Québec, CANADA - 5) Aarhus University Hospital, Department of Pediatrics, Aarhus N, DENMARK
PURPOSE
To evaluate the safety and efficacy of long-term treatment with solifenacin succinate oral suspension in children (5–11
yrs old) and adolescents (12–17 yrs old) with overactive bladder.
MATERIAL AND METHODS
119 children and 29 adolescents entered this 40-week extension study 2-3 days after the last dose of study medication
in the preceding 12-week, placebo-controlled study (52 weeks total). Solifenacin starting doses, based on subject’s
weight at screening, aimed to deliver steady-state plasma drug exposure equivalent to that delivered by a 5 mg dose in
adults (PED5). Titration was in steps of 3-weeks duration to attain optimal individual doses at Week 9 (final doses
equivalent to PED2.5, PED5, PED7.5 or PED10). Safety assessments were adverse events (AEs), ECG, vital signs and
post-void residual (PVR) volume. Efficacy variables (assessed using a 7-day diary) included change from baseline to end
of treatment in mean number of incontinence episodes/24 hrs and micturitions/24 hrs.
RESULTS
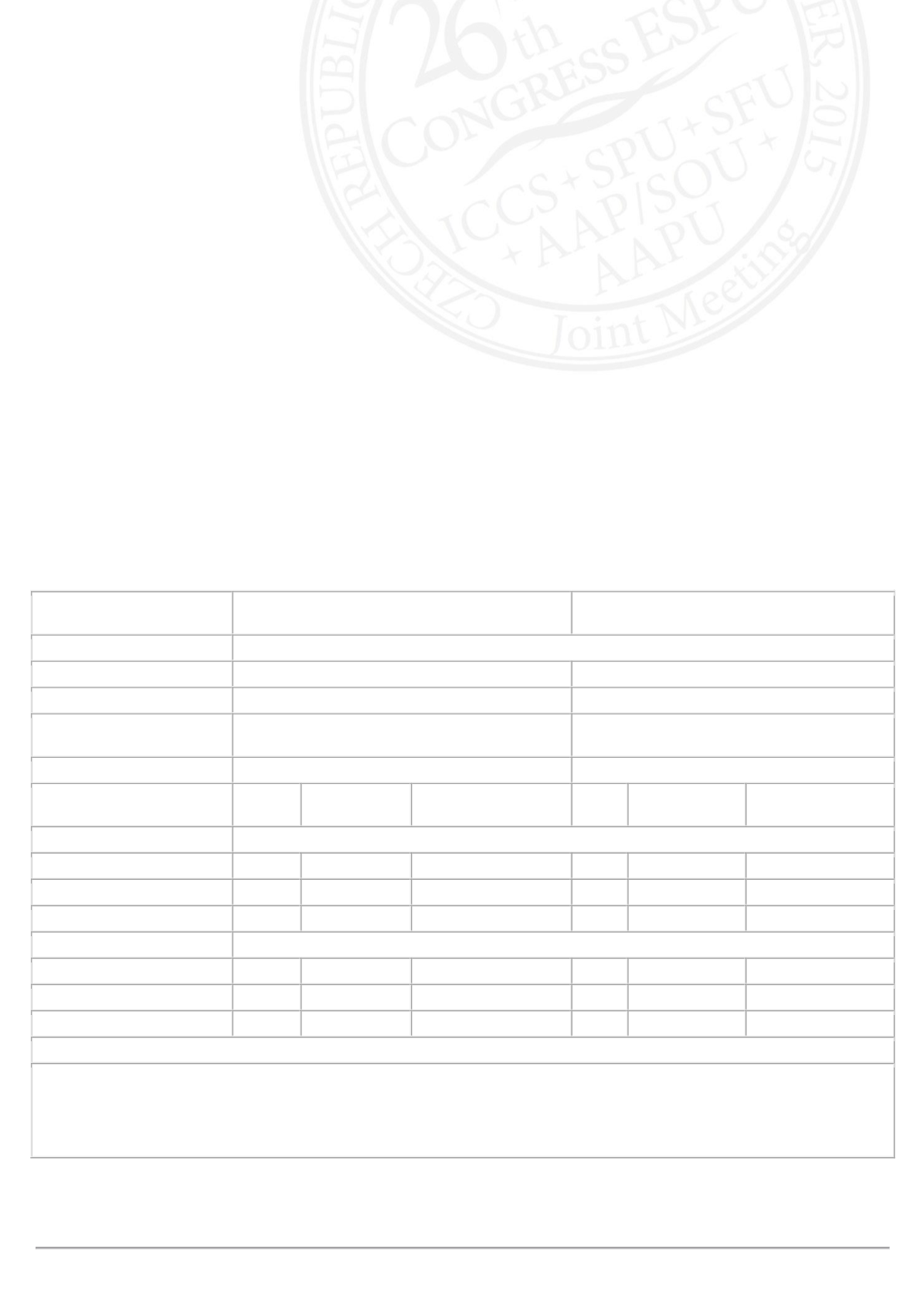
The most common drug-related treatment-emergent AEs are shown in the table; none were serious. Solifenacin did not
increase PVR volume and there were no apparent effects on vital signs or laboratory variables. Reductions in micturition
frequency and number of incontinence episodes/24 hrs that were evident after 3 weeks of treatment in the double-blind
study increased over the course of the open-label study up to 52 weeks.
Children (5–11 yrs)
n=118
Adolescents (12–17 yrs)
n=29
Incidence of most common drug-related TEAEs (SAF), n (%)†
Constipation
14 (11.9)
1 (3.4)
Nausea
0
2 (6.9)
Electrocardiogram QT
prolonged
10 (8.5)
4 (13.8)
Dry mouth
5 (4.2)
1 (3.4)
Duration of Solifenacin
Treatment
n
Mean (SD)
Mean Change From
Baseline (95% CI)*
n
Mean (SD)
Mean Change From
Baseline (95% CI)*
Mean number of incontinence episodes/24 hrs (FAS)
Baseline
117
2.7 (2.3)
29
2.7 (2.3)
40 weeks‡
97
1.1 (1.2)
–1.6 (–1.8, –1.3)
23
1.1 (1.8)
–1.6 (–2.3, –0.8)
52 weeks‡
44
1.0 (1.2)
–1.9 (–2.2, –1.7)
11
0.6 (1.0)
–2.0 (–2.8, –1.2)
Mean number of micturitions/24 hrs (FAS)
Baseline
117
8.2 (2.6)
29
8.0 (3.6)
40 weeks‡
97
6.8 (1.6)
–1.5 (–1.8, –1.2)
23
6.4 (1.6)
–1.2 (–1.9, –0.4)
52 weeks‡
44
6.6 (1.6)
–1.8 (–2.2, –1.4)
11
5.7 (1.1)
–1.8 (–2.6, –1.0)
*Adjusted estimates are from a repeated measures ANCOVA model
†TEAEs listed represent those with the highest incidence in both age cohorts combined
‡Total exposure to solifenacin is 40 weeks or 52 weeks depending on treatment allocation in the 12-week double-blind
study
CI=confidence interval, ECG=electrocardiogram, FAS=full analysis set, SAF=safety analysis set, SE=standard error,
TEAE=treatment-emergent adverse event
CONCLUSIONS
Solifenacin in a once-daily liquid formulation was well-tolerated in children and adolescents for up to 52 weeks of
exposure. Efficacy was maintained or increased relative to the preceding 12-week study.












